- Reading 5.1
- What evidence does the movie clip give you about the ability of the body to store food?
- Does this data support the claim that the body can store food for later use?
- Does all the weight gain suggest that the body is storing food for later use? What about weight gain as we grow? When is weight gain storage and when is it growing? What is BMI?
- Why do you think carbohydrates can/cannot be stored as fats? Does this mean a chemical reaction occurred? Why?
- If a person drinks many carbohydrates found in a soda pop, and eats carbohydrates (starch) found in french fries, but his/her body stores fat, what happened to those carbohydrate molecules? What evidence may be found in the movie?
Super Size Me: (clip: 7 minutes 21 seconds)
______________________________
bondingbasics08
MS-PS1-5: Chemical reactions and mass (ID#: 031.03-c07) http://authoring.concord.org/activities/5231/pages/69619/cface850-a023-4914-9ffe-29d29bad73c9
Chemical reactions and mass (ID#: 030.03-c07) http://authoring.concord.org/activities/5271/pages/70086/848673a7-107f-4d3e-8555-a525e2ff8e78
Also….Use Element Jeopardy and notes to undersatnd how matter is organized.
Relevant Terms:
| respiration | protein | enzymes | cholestrol | control |
| calories | glucose | fats | LDL | dependent variable |
| energy | Monosaccharides | oils | HDL | independent variable |
| chemical reactions | Polysaccharides | Triglcerides | reactants | constant |
| carbohydrates | nuclei acids | Saturated fats | products | |
| lipids | nutritionists | Trans fats | Reasoning using data | |
| Respiration: C6H12O6 + 6O2 —> CO2 + 6H2O + energy | ||||
| Photosynthesis: 6CO2 + 6H2O —> C6H12O6 + 6O2 | ||||
- Food provides energy and building materials for the cells.
- Different types of food molecules, when reacting with oxygen, produce different amounts of energy.
- The difference in the amount of energy that carbohydrates, proteins, and fats have the capacity to provide is due to the differences in the arrangement and number of different types of atoms in the molecules.
- Large food molecules are broken down through a chemical reaction.
- Organisms build up new molecules through a chemical reaction. These molecules are used for growth and repair. When food molecules are not used immediately, organisms can store them for short or long periods of time for energy or to be used as building materials.
- Plants need water and light to grow.
- Plants convert light energy into chemical energy.
- Photosynthesis is a chemical reaction that uses energy from the sun and the reactants of carbon dioxide and water to form glucose and oxygen.
- Burning food requires food molecules and oxygen and produces carbon dioxide and water. In the process, energy is released.
- Cellular respiration is the chemical reaction that provides energy to the cells of an organism. During cellular respiration, organisms (including plants and animals) use oxygen and food molecules and produce water and carbon dioxide.
- An ecosystem needs a constant input of light energy. As light energy enters the environment, plants use this for photosynthesis to create food molecules. These food molecules are used by plants and animals in cellular respiration to produce energy for cells.
- Carbon in the environment can cycle from food molecules to carbon dioxide through photosynthesis and cellular respiration. (During photosynthesis, plants rearrange the carbon atoms in carbon dioxide into carbon-containing food molecules. Then during cellular respiration, plants and animals rearrange the carbon in food molecules back into carbon dioxide.)
Soda Can Calorimeter
Energy Content of Food
Introduction:
Have you ever noticed the nutrition label located on the packaging of the food you buy? One of the first things listed on the label are the calories per serving. How is the calorie content of food determined? This activity will introduce the concept of calorimetry and investigate the caloric content of snack foods.
Concepts : Q = mC∆ T
- Calorimetry • Conservation of energy • First law of thermodynamics
In this experiment, the specific heat of water and its change in temperature will be used to determine the caloric content of a food sample. The normal unit for measuring the energy content in food is called a Calorie (with an uppercase C). A Calorie is really a kilocalorie, or 1000 calories (lowercase c). During calorimetry, food burns and its stored energy is quickly converted into heat energy and products of combustion (carbon dioxide and water). The heat energy that is released is then transferred into the water above it in the calorimeter. The temperature change in the water is then measured and used to calculate the amount of heat energy released from the burning food. The heat energy is calculated using Equation 1.
Materials
Balance (0.01-g precision) Snack foods (cheese puffs, popcorn, marshmallows, etc.)
Cork stopper Soda can, empty and clean
Matches, Stirring rod, glass, 3 to 4 large paper clips
Graduated cylinder, 100-mL Support stand
Metal ring with clamp Thermometer
Pin, large straight Water, distilled or tap, _200__ mL
Metric ruler
Safety Precautions
Wear safety glasses when performing this or any lab that uses chemicals, heat or glassware. Care should be taken when handling or placing food onto the pin point. Allow the food sample to cool before touching or discarding it. Use a glass stirring rod to stir the liquid; never stir with a thermometer. Students should not be allowed to eat the snack foods once they are brought into the lab. This lab should be performed in a well-ventilated room. Wash hands thoroughly with soap and water before leaving the laboratory.
Flinn Scientific Publication No. 10861
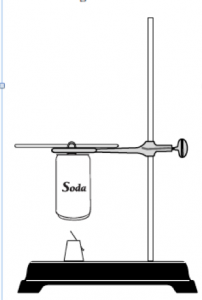

Procedure
- Push the pin through the cork so that the pin head is flush with the cork. If the pin is large enough, try to go through the center. If this is hard to do, try to insert the pin at an angle through the side and top of the cork. See Figure 1. Note: This setup will now be referred to as the “Food Holder.”
- Place a food sample on the food holder. Measure and record the combined mass of the food holder and sample. Place the food holder on the base of a support stand.
- Using a graduated cylinder, measure and add 50.0 mL of water to an empty, clean soda can.
- Bend the tab on the soda can and slide a glass stirring rod through the hole. Suspend the can on a support stand using a metal ring. See Figure 2. Adjust the height of the can so that it is about 2.5 cm above the food holder.
- Insert a thermometer into the can. Measure and record the initial temperature of the water.
- Light the food sample and center it under the soda can. Allow the water to be heated until the food sample stops burning. Record the maximum (final) temperature of the water in the can.
- Measure and record the final mass of the food holder and sample.
- Clean the bottom of the can and remove any food residue from the food holder.
- Repeat steps 1–8 two more times with provided different snack food samples.
October 28—
RAD Fundraising results include:
No additional homework being given Oct. 28th – Nov. 2nd 2016
2 Activity half days
October 24, 2016
Period 5 Red and White Days–Advanced Topics–
Homework:
- Complete Textbook Pg. 15 Making Sense #1 – #3
- Article placed in your folder
- Research for problem identified and draft hypothesis written.
NOTES:
Using the respiration reaction:
6O2 + C6H12O6 —> 6CO2 + 6H2O + energy
We are using class data:
| CO2 (ppm) | 10/20/16 | 10/24/16 | |||
| Meter 1 | 725 | 720 | 1018 | 1005 | 1005 |
| Meter 2 | 699 | 688 | 1081 | 1081 | 1071 |
| Meter 3 | 679 | 692 | 1049 | 1064 | 1049 |
| O2 | ————– | 1.3 mg/L | 1.2 mg/L | ||
Student’s Science Project Finds Toilet Water Cleaner Than Restaurant Ice.
A seventh-grade student in Florida was awarded first place for her science fair project that found some toilet water is cleaner than ice served at area restaurants, according to a Local 6 News report.
Student Jasmine Roberts checked five fast-food restaurants near the University of South Florida and found there was more bacteria in the ice people were drinking than there was in the same restaurants’ toilet water.
“When I get ice, sometimes I order ice just to chew on it, and now I know I’m not going to do that anymore just because of the amount of bacteria I found,” Roberts said.
Since the Benito Middle School student completed the project, she has received international attention.
Dr. Daniel Lim, who is a microbiology professor at USF, said he was not surprised the toilet water had less bacteria than restaurant ice.
If you don’t clean the receptacle, the materials routinely, there may be a possibility of biofilm or residual material remaining in that receptacle,” Lim said.
Jim Griffith, the president of Suncoast Ice Machines, sells, leases and services ice machines. He also wondered about the cleanliness of the ice dispensers the students sampled.
The Florida Department of Business and Professional Regulation checks ice machines during restaurant inspections twice a year.
Roberts won $800 for the project.
Let’s use Jasmine’s experiment as an example for our problem solving focus:
“My hypothesis was that the fast food restaurants’ ice would contain more bacteria that the fast food restaurants’ toilet water.”
After testing water from the toilets and ice taken from 5 area fast food restaurants, her conclusion:
“I found that 70-percent of the time, the ice from the fast food restaurant’s contain more bacteria than the fast food restaurant’s toilet water.”
4 Parts of a Scientific Experiment: (Textbook pages 754 & 755)
- Independent Variable—What is measured; the manipulated variable
- Dependent Variable —Results, data
- Constant——————Conditions of the experiment that do not change
- Control——————-Used for comparison to independent variable
Placebo—-a fake independent variable to understand the effect of just using the independent variable
October11
- Use Scientific Method to write an experiment for a problem important to you.
- Select an article about a topic you want to read and know more about. Use:
https://www.sciencenewsforstudents.org/ Or
https://www.scientificamerican.com/
September 28,and 29 2016
Assignment/Planner: Read 1.1 from the textbook and fill in sections when asked questions or what you think. Due 10/3 (W) and 10/4(R)
Consider:
__________________
When you see a video in class:
Remember: Write your reaction and new information while listening so as not to distract others.
______________
September 27, 2016
Assignment: (Read assignment before starting)
Use your provided log in (username and password) while and class and at outside of class to access your digital editable textbook at :
http://eugene4j.iqwst.com/webapp/
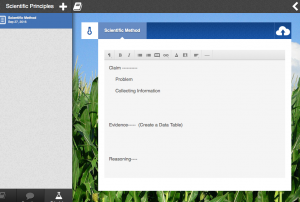
I. Be sure to open you textbook and add your First entry: Scientific Method Practice. In the body of the message include:
Claim–Problem, Materials, Procedure
Evidence–Write and Do an Experiement–
- Make a data table and record data
Reasoning—Think about and analyze
- Communicate Results
II. Make a drawing of what you saw in class using the “paint brush option”
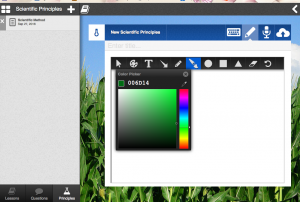
Save both each time by selecting the cloud with the arrow in the upper right corner.
______________
In addition to the Safety Rules in your notes you copied in class :
- Leave the lab area and equipment in the proper place at the end of lab.
- Wash your hands before leaving the lab.
- Return lab material to proper places after use
- Wash used glassware and instruments
- Wipe off work areas
- Clean the sink after use
9/23/16
Students should be able to get into their digital resources portal using instructions from class.
Study for quiz next week
Period 5 Handout:
The Ocean Exploration Trust was founded in 2008 by Titanic-discoverer and National Geographic Explorer-in-Residence Dr. Robert Ballard to engage in pure ocean exploration. Our international programs center on scientific exploration of the seafloor and many of our expeditions are launched from aboard Exploration Vessel (E/V) Nautilus, a 64-meter research vessel operated by the Ocean Exploration Trust. In addition to conducting scientific research, we offer our expeditions to explorers on shore via live video, audio, and data feeds from the field.
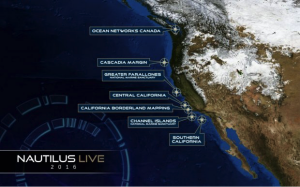
Mapping the California Borderland August 29, 2016 to September 12, 2016
For the final leg of the 2016 expedition, Nautilus will work along the California
For the final leg of the 2016 expedition, Nautilus will work along the California Borderland region offshore San Diego to San Francisco. In this complex margin within America’s Exclusive Economic Zone, few areas of the seafloor have been extensively mapped. E/V Nautilus will utilize its hull-mounted multibeam echosounder to survey these zones and create maps to show the acoustically-derived bathymetry of the seafloor.
Although only about 10% of our world’s oceans have been acoustically mapped at, satellites equipped with altimetry sensors have been used to derive the bathymetry of the entire seafloor. The altimeters sense gravity anomalies of the sea surface that can be linked to topography (e.g. dip in the surface of the ocean over a trench). There is a tradeoff between bathymetry derived from altimetry versus shipboard acoustic sensors: multibeam systems map the seafloor at a high resolution and are accurate, but ships move slowly only having mapped about 1/10th of the seafloor. While there is global coverage from satellite altimetry-derived maps, the resolution of these maps is low, and the correlation between depth and a gravity anomaly is non-linear (meaning there is more room for error deriving bathymetry from satellite measurements).
1. Googly Eyed Cutie Squid http://www.nautiluslive.org/video/2016/08/12/googly-eyed-stubby-squid
Robert Ballard
Director of the Center for Ocean Exploration, Graduate School of Oceanography URI
President of the Ocean Exploration Trust
Among the most accomplished and well known of the world’s deep-sea explorers, Dr. Robert Ballard is best known for his historic discoveries of hydrothermal vents, the sunken R.M.S. Titanic, the German battleship Bismarck, and numerous other contemporary and ancient shipwrecks around the world. During his long career he has conducted more than 150 deep-sea expeditions using the latest in exploration technology.
Dr. Ballard has been a pioneer in the development of advanced deep submergence and telepresence technology. Although his Ph.D. is in Marine Geology and Geophysics, his scientific interests run the gamut from the volcanic, tectonic, and hydrothermal processes of the mid-ocean ridge to deep-sea archaeology and maritime history. Dr. Ballard also spends a great deal of his time involved in various educational outreach programs. In 2008, Dr. Ballard secured the E/V Nautilus, which has become his flag-ship for exploration, operated by the Ocean Exploration Trust and funded in part by NOAA’s Office of Ocean Exploration. Nautilus is connected by way of a high bandwidth satellite link to the University of Rhode Island’s Inner Space Center and from there to the world.
“As a child I was always curious about things and I was fortunate enough not to have that passion extinguished as I grew up. E/V Nautilus and the Ocean Exploration Trust give me the opportunity to pour fuel on the flames of the public’s curiosity to help keep it alive for them.”
Stephanie Martínez Rivera
Ocean Science Intern
Graduate Student
University of Maryland Eastern Shore
Tell us about your work / research. What kinds of things do you do?
I am a graduate student at the University of Maryland Eastern Shore. Currently, I am in my 3rd year of the Ph.D. program in Marine Estuarine-Environmental Science with a focus on Fisheries Science. My research focuses on the reproductive biology of the deep-sea red crab, which has supported a federally-managed data-poor fishery in Mid-Atlantic and southern New England since the 1970’s. Our main goal is to glean all the information we possibly can in order to improve their management strategies, and expand our knowledge on deep-sea decapods. The main challenge with this species is the limited information about their biology, abundance, growth, age, reproduction, and habitats, as well as the difficulty of collecting samples. One of the aspects of my research that I enjoy the most is the five day boat trips with the Atlantic Red Crab Company aboard the F/V Hannah Boden.
What sparked your initial interest in your career?
I grew up on a Caribbean island full of marine and coastal ecosystems and resources. Puerto Rico, the enchanted island, is an archipelago located between the North Atlantic Ocean and the Caribbean Sea. Since I was 5 years old, my father, a recreational fisherman, used to take me to the beach every weekend. My family and I spent our days at the beach swimming, fishing, snorkeling, or just enjoying the spectacular view. I remember being extremely intrigued with the wonders of the ocean, and I still am. My curiosity prompted in me the desire to work as a volunteer to protect our oceans. Consequently, I participated in educational nature walks, beach clean-ups, mangrove forest explorations, and workshops. As I grew older, my passion for the ocean progressed into my study of Marine Biology.
What element of your work / study do you think is the most fascinating?The most fascinating element of my work is diving into the unknown, making new discoveries, and further the knowledge of deep-sea biology. In addition, in the past years I have participated in three research cruises as a scientist intern which has been an extremely fulfilling experience. Ocean exploration is fascinating!
Jonathan Zand
Argus Pilot
ROV Pilot and Engineer
Ocean Dynamics Inc.
Jonathan Zand is a Professional Engineer and ROV pilot with a history of operating, maintaining, developing and deploying oceanographic equipment. Jonathan maintains, pilots, and navigates ROVs and small vessels for Ocean Dynamics Inc. in British Columbia, Canada. He also worked as a Systems Integration Engineer at Ocean Networks Canada providing technical expertise for the installation and operation of the NEPTUNE cabled observatory west of Vancouver Island.
Jonathan Zand graduated from the University of British Columbia in 2005 with a degree in Mechanical Engineering. He later completed a Masters of Applied Science with focus on ROV navigation at the University of Victoria in 2009.
“I love working with ROVs and using them to explore the ocean.”
Mallory Ringham
Science/Data Team
Graduate Student
MIT/WHOI Joint Program
Tell us about your work/ research. What kinds of things do you do?
Recently, I completed an MS in Earth Sciences at Syracuse University, where I worked on pedogenic carbonate in desert soils of the central Andes in Argentina and Chile. My thesis project involved environmental ground-truthing of the clumped isotope geothermometer, which is a proxy for estimating near-surface temperatures used in paleoenvironmental and paleoaltitudinal studies. Soon, I will be trading in mountains and deserts for coastal and marine environments. I am beginning my Ph.D. studies in the MIT/WHOI Joint Program this fall, where I will work on carbonate chemistry and the inorganic carbon cycle in marine systems.
What sparked your initial interest in your career?
I started out in physics and chemical engineering as an undergraduate, but after a few courses in oceanography and biogeochemistry, I decided to put both of my majors to use on highly interdisciplinary earth sciences projects in graduate school. My Master’s project up in the Andes mountain range was a great excuse to get outside and travel while contributing to interesting paleoenvironmental science. Joining a Ph.D. program in oceanography will allow me to continue exploring the Earth while putting my science and engineering background to great use.
What element of your work/ study do you think is the most fascinating?
It’s incredible to see that scientific research can take us far outside of our laboratories. I have worked with scientists, engineers and educators from around the world while conducting field work in the Andes and while sailing around on the E/V Nautilus. The connections and knowledge that we share with others both in and outside of our scientific fields are invaluable.
Embed code: How to Cough and Sneeze Hygenically :
<iframe src=”http://pisdtv.pisd.edu/embed/O_iad_w93ed1UzUxwjwT” width=”500″ height=”420″ frameborder=”0″ scrolling=”no”></iframe>
Save
Save
Save
Save
Save
Save
Save
Save
Save
function googleTranslateElementInit() {
new google.translate.TranslateElement({pageLanguage: ‘en’, layout: google.translate.TranslateElement.InlineLayout.HORIZONTAL}, ‘google_translate_element’);
}
Save
Save
Save
No comments